Ammonia-modified Co(ii) sites in zeolites: IR spectroscopy and spin-resolved charge transfer analysis of NO adsorption complexes†
Abstract
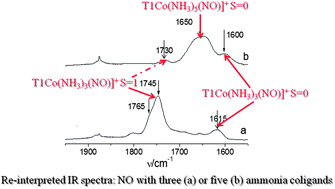
IR spectroscopic studies and quantum chemical modeling (aided by the analysis of charge transfer processes between co-adsorbed ammonia and the Co(II)–NO adduct) evidence that donor ammonia molecules, ligated to extraframework Co2+ centers in zeolites, vitally affect the strength of the N–O bond. Calculations indicate that versatility of ammine nitrosyl complexes, differing in the number of NH3 ligands as well as in the geometry and electronic structure of the Co–N–O unit (showing variable activation of NO) may co-exist in zeolite frameworks. However, only combined analysis of experimental and calculation results points to the adducts with three or five NH3 coligands as decisive. The novel finding concerning the interpretation of discussed IR spectra is the assignment of the most down-shifted bands at 1600–1615 cm−1 to the N–O stretch in the singlet [Co(NH3)3(NO)]2+ adduct, in place of tentative ascription to pentaammine adducts. Theory indicates also that the Co(II) center (with manifold of close-lying electronic and spin states) acts as a tunable electron donor where the spin state may open or close specific channels transferring electron density from the donor ligands (treated as the part of environment) to the NO molecule.
- This article is part of the themed collection: Porous Materials (FEZA 2014)

 Please wait while we load your content...
Please wait while we load your content...