Propene epoxidation with O2 or H2–O2 mixtures over silver catalysts: theoretical insights into the role of the particle size†
Abstract
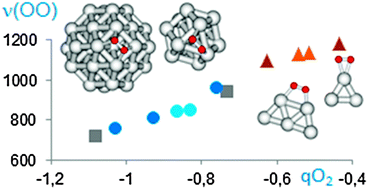
The adsorption and dissociation of molecular O2 over silver extended surfaces, nanoparticles and clusters of different size, as well as the reaction between adsorbed O2 and H2 to form less nucleophilic hydroperoxide groups have been investigated by means of periodic DFT calculations. Hydroperoxide formation from O2 and H2 is in all systems kinetically favoured over O2 dissociation, which becomes energetically forbidden on small planar clusters. The nature and reactivity towards propene of all oxygen species present on silver, including adsorbed molecular O2, atomic O, and hydroperoxide groups, have been theoretically explored. Formation of the oxametallacycle intermediate involved in propene epoxidation is energetically accessible on subnanometric three-dimensional silver nanoparticles, but competitive pathways leading to hydrogen abstraction and allyl formation always involve lower activation barriers. Theoretical findings have been experimentally confirmed by Raman spectroscopy of O2 adsorption and catalytic testing of planar and three dimensional silver clusters.
- This article is part of the themed collection: Size Selected Clusters and Particles: From Physical Chemistry to Catalysis

 Please wait while we load your content...
Please wait while we load your content...