Catalytic oxidation of CO with N2O on isolated copper cluster anions†
Abstract
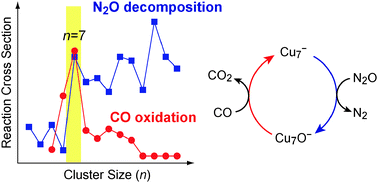
A catalytic redox reaction involving N2O and CO on size-selected copper cluster anions, Cun−, was investigated in the gas phase using a guided ion-beam tandem mass spectrometer. When Cun− is exposed to a mixture of N2O and CO, CunO− is produced via the decomposition of N2O. Increase of the CO partial pressure results in the reproduction of Cun− and decrease of CunO− through the oxidation of CO. The present results demonstrate that a full catalytic cycle for the reaction, N2O + CO → N2 + CO2, takes place on copper cluster anions. Furthermore, in the investigations of the elementary reactions of Cun− + N2O and CunO− + CO, we found that the catalytic oxidation of CO with N2O on Cun− proceeds most efficiently at n = 7 in the size range of n = 5–16.
- This article is part of the themed collection: Size Selected Clusters and Particles: From Physical Chemistry to Catalysis

 Please wait while we load your content...
Please wait while we load your content...