Concerted charge and energy transfer processes in a highly flexible fullerene–dye system: a mixed quantum–classical study
Abstract
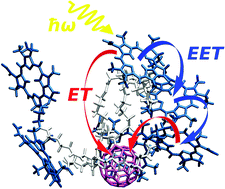
Photoinduced excitation energy transfer and accompanying charge separation are elucidated for a supramolecular system of a single fullerene covalently linked to six pyropheophorbide-a dye molecules. Molecular dynamics simulations are performed to gain an atomistic picture of the architecture and the surrounding solvent. Excitation energy transfer among the dye molecules and electron transfer from the excited dyes to the fullerene are described by a mixed quantum–classical version of the Förster rate and the semiclassical Marcus rate, respectively. The mean characteristic time of energy redistribution lies in the range of 10 ps, while electron transfer proceeds within 150 ps. In between, on a 20 to 50 ps time-scale, conformational changes take place in the system. This temporal hierarchy of processes guarantees efficient charge separation, if the structure is exposed to a solvent. The fast energy transfer can adopt the dye excitation to the actual conformation. In this sense, the probability to achieve charge separation is large enough since any dominance of unfavorable conformations that exhibit a large dye–fullerene distance is circumvented. And the slow electron transfer may realize an averaging with respect to different conformations. To confirm the reliability of our computations, ensemble measurements on the charge separation dynamics are simulated and a very good agreement with the experimental data is obtained.

 Please wait while we load your content...
Please wait while we load your content...