Oxygen-evolving complex of photosystem II: correlating structure with spectroscopy
Abstract
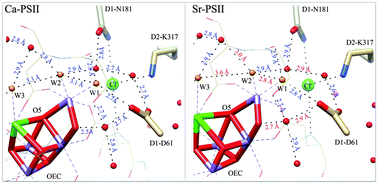
Water oxidation at the oxygen-evolving complex (OEC) of photosystem II (PSII) involves multiple redox states called Sn states (n = 0–4). The S1 → S2 redox transition of the OEC has been studied extensively using various forms of spectroscopy, including electron paramagnetic resonance (EPR) and Fourier transform infrared (FTIR) spectroscopy. In the S2 state, two isomers of the OEC are observed by EPR: a ST = 1/2 form and a ST = 5/2 form. DFT-based structural models of the OEC have been proposed for the two spin isomers in the S2 state, but the factors that determine the stability of one form or the other are not known. Using structural information on the OEC and its surroundings, in conjunction with spectroscopic information available on the S1 → S2 transition for a variety of site-directed mutations, Ca2+ and Cl− substitutions, and small molecule inhibitors, we propose that the hydrogen-bonding network encompassing D1-D61 and the OEC-bound waters plays an important role in stabilizing one spin isomer over the other. In the presence of ammonia, PSII centers can be trapped in either the ST = 5/2 form after a 200 K illumination procedure or an ammonia-altered ST = 1/2 form upon annealing at 273 K. We propose a mechanism for ammonia binding to the OEC in the S2 state that takes into account the hydrogen-binding requirements for ammonia binding and the specificity for binding of ammonia but not methylamine. A discussion regarding the possibility of spin isomers of the OEC in the S1 state, analogous to the spin isomers of the S2 state, is also presented.
- This article is part of the themed collection: Photosynthesis: From Natural to Artificial

 Please wait while we load your content...
Please wait while we load your content...