Exploring the structure–aromaticity relationship in Hückel and Möbius N-fused pentaphyrins using DFT†
Abstract
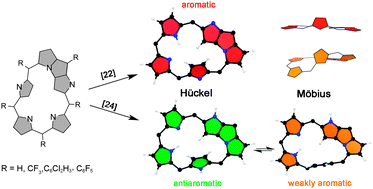
N-fused pentaphyrins (NFP) are the stable forms of fully meso-aryl pentaphyrins(1.1.1.1.1). In order to determine the optimum conditions for viable Möbius topologies of these porphyrinoids, the conformational preferences, Hückel–Möbius interconversion pathways and aromaticity of [22] and [24]NFP have been investigated using density functional theory calculations. The conformation of the macrocycle is shown to be strongly dependent on the oxidation state and the macrocyclic aromaticity. [22]NFP prefers a highly aromatic and relatively strain-free Hückel conformation. However, antiaromatic Hückel and weakly aromatic Möbius conformers coexist in dynamic equilibrium in [24]NFP. The Hückel–Möbius aromaticity switch requires very low activation energy barriers (Ea = 3–4 kcal mol−1). Interestingly, the balance between Möbius and Hückel conformations in [24]NFP can be controlled by meso-substituents. The structure–property relationship between the molecular conformation, number of π electrons and aromaticity has been established in our study using energetic, magnetic, structural, and reactivity descriptors of aromaticity. Although the Möbius topology is indeed accessible for [24]NFP, it does not exhibit a distinct macrocyclic aromaticity mainly due to the large dihedral angles around the molecular twist. Regarding the computational methodology, B3LYP and M06 show the best overall performance for describing the experimental geometries of NFP and, importantly, our computational results support the experimental evidence available for N-fused pentaphyrins.
- This article is part of the themed collection: Density functional theory and its applications

 Please wait while we load your content...
Please wait while we load your content...