Using the reaction force and the reaction electronic flux on the proton transfer of formamide derived systems
Abstract
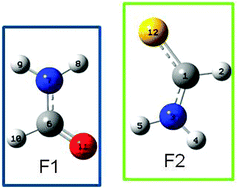
In this work, we present a theoretical study of the mechanism of double proton transfer in formamide, formamide–thioformamide and thioformamide dimers. The reaction mechanisms were analyzed in terms of the energy profile and novel concepts such as the reaction force profile and reaction electronic flux, along with local electronic properties such as NBO analysis. All systems were characterized computationally using DFT/B3LYP 6-311G** on Gaussian09. These results show that the electronic processes take place mostly in the transition state for all the systems; in the formamide and thioformamide dimers, electron transfer has a synchronic nature, while the electron transfer is asynchronic in the formamide–thioformamide dimer.
- This article is part of the themed collection: Density functional theory and its applications

 Please wait while we load your content...
Please wait while we load your content...