Electrochemical activation of pristine single walled carbon nanotubes: impact on oxygen reduction and other surface sensitive redox processes
Abstract
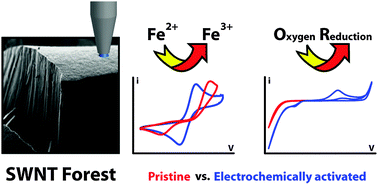
The effect of systematic anodic pre-treatments of pristine single walled carbon nanotube (SWNT) forests on the electrochemical response towards a variety of redox processes is investigated. An experimental arrangement is adopted whereby a microcapillary containing the solution of interest and a quasi reference-counter electrode is brought into contact with a small portion of the forest to enable measurements on the surface before and after controlled anodic polarisation (AP). AP of the surface is found to both improve the voltammetric response (faster apparent heterogeneous electron transfer kinetics) of surface sensitive redox processes, such as Fe2+/3+, and enhance the electrocatalytic response of the SWNTs towards oxygen reduction; the extent of which can be carefully controlled via the applied anodic potential. AP is expected to remove any trace organic (atmospheric) contaminants that may accumulate on the forest over extended periods as well as allowing the controlled introduction of defects, as confirmed by micro-Raman spectroscopy.
- This article is part of the themed collection: PCCP’s 15th anniversary

 Please wait while we load your content...
Please wait while we load your content...