1D, 2D and 3D coordination polymers of 1,3-phenylene diisonicotinate with Cu(i)/Cu(ii): Cu2I2 building block, anion influence and guest inclusions†
Abstract
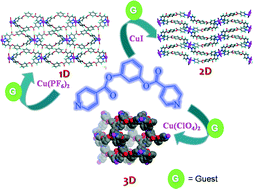
The reactions of a flexible bidentate ligand 1,3-phenylene diisonicotinate (L) with Cu(I/II) salts afforded coordination polymers with varied dimensionalities and guest inclusion capabilities. Complexation of L with CuI resulted in a 2D-network with (4,4) topology and inclusion of guest molecules such as CHCl3, bromobenzene, nitrobenzene and benzonitrile. The treatment of L with Cu(PF6)2 and Cu(ClO4)2 resulted in a one-dimensional network containing M2L2 macrocycles and a 4-fold interpenetrated three dimensional network having quartz topology, respectively. The 1D-networks included CHCl3 as guests and the interpenetrated 3D-network includes tetrahedral water clusters.
- This article is part of the themed collection: International Year of Crystallography Celebration: India

 Please wait while we load your content...
Please wait while we load your content...