Use of the oxime–oximato binding mode to stabilise mixed valence copper iodide polymer networks using dipyridyl ketone oxime ligands†
Abstract
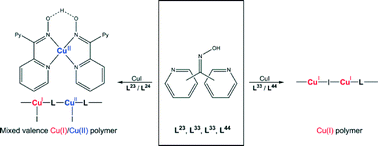
Five complexes prepared from CuI and dipyridyl ketone oxime ligands were synthesised and structurally characterised, [Cu2I2(L23)(L23-H)]∞, 1, [Cu(L23-H)2]∞, 2, {[Cu2(L24)(L24-H)I2](CH3CN)}∞, 3, [Cu(L33)I]∞, 4, and {[Cu8(L44)5I8](CH3CN)4(C4H10O)2(H2O)2}∞, 5. The effect of the varying pyridyl substitution patterns of L23, L24, L33 and L44 was investigated, as well as the effect this had on forming mixed valence complexes. Complexes 1 and 3 formed mixed valence Cu(I)/Cu(II) polymer networks with Cu(II) stabilised by the formation of a pseudo tetradentate ligand through an oxime–oximato bridge. In comparison, the Cu(I) in complex 2 was completely oxidised to Cu(II), with the ligand fully deprotonating to act as a charge balance. Complex 4 formed a simple 1D Cu2I2 rhomboid dimer daisy chain, while 5 formed Cu4I4 cubane tetramer clusters, allowing four ligands to bind to one centre. Overall 5 existed as two interpenetrated hydrogen bonded 3D networks.
- This article is part of the themed collection: International Year of Crystallography Celebration: Asia-Pacific

 Please wait while we load your content...
Please wait while we load your content...