Comparison of pyridyl and pyridyl N-oxide groups as acceptor in hydrogen bonding with carboxylic acid†
Abstract
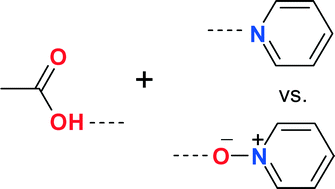
Competition experiments between pyridyl and pyridyl N-oxide groups have been performed to find out which of these two groups is a better acceptor in hydrogen bonding with the carboxylic acid group. 4,4′-bipyridine N-monoxide, a rigid, conjugated, and linear molecule and 4,4′-trimethylenebipyridine N-monoxide, a flexible, non-conjugated between two aryl groups, and bent molecule, have been used to synthesize complexes with some carboxylic acid containing compounds. The study shows that although the occurrence of pyridyl⋯acid synthon is more than the corresponding pyridyl N-oxide⋯acid synthon, based on the distance criterion and energy calculation, the pyridyl N-oxide⋯acid synthon is slightly stronger than the pyridyl⋯acid synthon. Solubility studies also show that the pyridyl N-oxide compound may be a better choice as a coformer than the corresponding pyridyl derivative to increase the solubility of carboxylic acid containing compounds.
- This article is part of the themed collection: International Year of Crystallography Celebration: India

 Please wait while we load your content...
Please wait while we load your content...