Hydrogen bond synthon competition in the stabilization of theophylline cocrystals†
Abstract
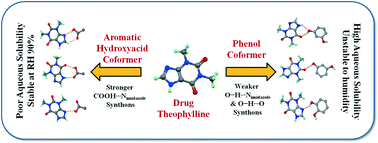
Theophylline medications are used as bronchodilators that relax the muscles in the breathing tubes. Issues relating to the hydration behaviour and moisture stability of this drug are well-known. Seven new cocrystals of theophylline with phenols, and isomeric hydroxybenzoic acids are synthesized. Solvent assisted mechanochemical synthesis is employed in order to synthesize the cocrystal materials. Single crystal structures are obtained for all cocrystals and furthermore they are characterized thermally, spectroscopy and by powder X-ray diffraction. The hydrogen bonding and crystal packing features are examined and found to be comparable to reported cocrystals/salts of theophylline or its molecular analogues, i.e. caffeine and theobromine. The solubility of these cocrystals are determined using UV-vis spectroscopy and correlated with the hydrogen bond synthon energies computed on Gaussian using DFT. The occurrence probability of the hydrogen bond synthons are examined with the Cambridge Structural Database (CSD). An important observation is that the phenol coformers facilitate water assimilation in the crystalline lattice since they offer a weaker O–H⋯Nimidazole H bond synthon over the stronger COOH⋯Nimidazole H-bond available in hydroxybenzoic acid coformers. The presence of the –COOH group prevents water incorporation and provides added physical stability to the cocrystal at highly humid conditions. The results show the feasibility of cocrystal design of a drug to tune its physical properties based on hydrogen bond synthons.
- This article is part of the themed collection: International Year of Crystallography Celebration: India

 Please wait while we load your content...
Please wait while we load your content...