Investigation of molecular arrangements and solid-state fluorescence properties of solvates and cocrystals of 1-acetyl-3-phenyl-5-(9-anthryl)-2-pyrazoline†
Abstract
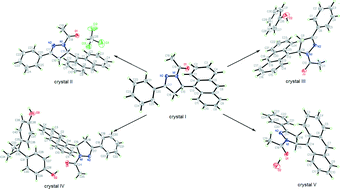
The title compound (APAP) was crystallized under different conditions to afford five types of crystals: the guest-free crystal (I), APAP·chloroform solvate (II), the APAP·phenol cocrystal (III), the APAP·2,2-bis(4-hydroxyphenyl)propane cocrystal (IV) and monohydrate (V). Single-crystal X-ray analysis revealed that a racemic chain motif is formed by the heterochiral APAP molecules in the guest-free crystal (I), in which anthracene fluorophores adopt a face-to-face π-stacked arrangement. In contrast, by entrapment of different guest molecules, the homochiral chains are formed by one-handed enantiomers in crystals II–V, in which no effective π-overlap exists between anthracene fluorophores. The optical-physical properties of crystals I–IV and their structure–property relationship were investigated. Crystal I exhibits a broad emission band with only one peak centered at 458 nm. However, crystals II–IV exhibit similar fluorescence spectra, in which the vibrational features are in good agreement with that of anthracene monomer emission and the λem are 430, 429 and 425 nm. As a control experiment, we speculate that the π-stacked arrangement of anthracene fluorophores should be responsible for larger red-shifted emission, lower emission quantum yield and longer lifetime. This strategy based on regulating the packing modes of anthracene fluorophores to tune the fluorescence properties and the investigation of structure–property relationships should be useful for the development of organic solid materials.
- This article is part of the themed collection: Functional Co-crystals

 Please wait while we load your content...
Please wait while we load your content...