Crystal growth by oriented attachment: kinetic models and control factors
Abstract
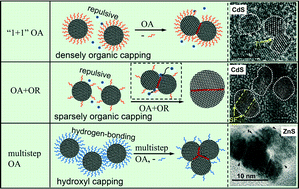
Crystal growth mechanism, kinetics, and microstructure development play fundamental roles in tailoring materials with controllable sizes and morphologies. Oriented attachment (OA) involves the spontaneous self-organization of adjacent nanocrystals, resulting in crystal growth by addition of solid particles that share a common crystallographic orientation. It is common for crystal growth to occur by more than one mechanism simultaneously, such as OA and coarsening. This complexity leads to the difficulty in studying the OA growth mechanism. Here, we briefly review progress in kinetic models involving OA and the impact of this mechanism on materials science. We concentrate mostly on recent findings that relate different OA behaviors to surface chemistry and growth conditions, aiming to elucidate this crystal growth mechanism. To explore OA-limited growth, the influence of the Ostwald ripening (OR) mechanism needs to be known and quantified. The introduction of capping ligands has been reported to play multiple roles, including i) promoting or inhibiting aggregation and thus influencing OA growth, or ii) hindering OR from occurring and thus facilitating OA. A detailed survey of nanocrystal growth kinetics under the effect of surface adsorption is presented and summarized.
- This article is part of the themed collection: Nanocrystal growth via oriented attachment

 Please wait while we load your content...
Please wait while we load your content...