Anion-tunable control of thermal Z→E isomerisation in basic azobenzene receptors†
Abstract
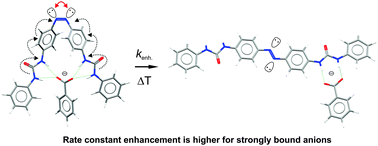
Herein, we report that thermal Z→E isomerisation of simple azobenzene urea derivatives is selectively and predictably controlled by anion binding. The rate of this process depends strictly on the anion concentration and its binding affinity to the Z-isomer of the azobenzene host, i.e. increased rate constants are observed for higher anion concentration as well as for more strongly bound guests. The origin of this phenomenon is attributed to the electron density transfer from the anion to the host π-system, resulting in increased repulsion between the lone electron pairs in the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond.
N bond.

 Please wait while we load your content...
Please wait while we load your content...