Metal-free oxidative decarbonylative halogenation of fused imidazoles†
Abstract
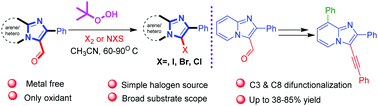
An efficient strategy has been developed for the deformylative halogenation of carbaldehyde imidazo-fused heterocycles in the presence of TBHP controlled by temperature. A convenient and sequential functionalization (C8 to C3) portrays the synthetic utility of the current method. N-Heterocycle benzamide products were also observed via the ring opening of imidazopyridines through the cleavage of C–C bond at high temperatures. Features of this method include temperature-controlled excellent regioselectivity, mild conditions and functional group tolerance.


 Please wait while we load your content...
Please wait while we load your content...