Experimental and theoretical investigations on Se(iv) and Se(vi) adsorption to UiO-66-based metal–organic frameworks†
Abstract
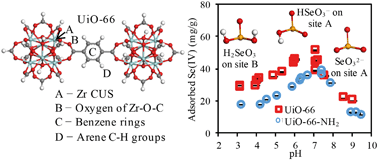
Adsorption behaviors and mechanisms of selenite [Se(IV)] and selenate [Se(VI)] on two types of zirconium metal–organic frameworks (MOFs, UiO-66 and UiO-66-NH2) were investigated using batch experiments, spectroscopic analyses, and theoretical calculations. Se(IV) adsorption was substantially greater than Se(VI) adsorption. Intriguingly, with increasing solution pH, Se(IV) adsorption increased to a maximum at pH 7, whereas Se(VI) adsorption monotonically decreased. Se(IV) adsorption was not influenced by coexisting ions except for PO43−, whereas Se(VI) adsorption was in general decreased with increasing coexisting ion concentrations. The underlying molecular binding mechanisms were elucidated via zeta potential, XPS, and FTIR analyses as well as density functional theory calculations. Se(IV) formed a strong inner-sphere complex with the MOFs via Lewis acid/base complexation between the Zr coordinatively unsaturated sites and HSeO3− or SeO32− (−59.4 to −142 kJ mol−1) and hydrogen bonding between Zr–O–C and H2SeO3 (−24.4 to −29.8 kJ mol−1). Se(VI) formed outer-sphere complexes with the MOFs through electrostatic interactions (−50.6 to −62.1 kJ mol−1). Thus, the BET surface area was the predominant factor determining the Se(IV) adsorption, whereas both the surface charge and BET surface area controlled the Se(VI) adsorption. This study provided new insights on the molecular interactions between Se(IV) and the MOFs, and revealed the distinct differences between the Se(IV) and Se(VI) adsorptions.


 Please wait while we load your content...
Please wait while we load your content...