A comparative study on the oxidation of two-dimensional Ti3C2 MXene structures in different environments
Abstract
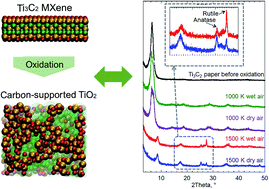
The oxidation of two-dimensional Ti3C2 MXene structures has been recognized as a promising method for the formation of hybrid structures of carbon supported nanotitania. Here, we studied the oxidation of Ti3C2 MXene structures using reactive force field (ReaxFF) based molecular dynamics simulations. To investigate the effect of oxidizing agent, we used three different environments including dry air (oxygen molecules), wet air (oxygen and water molecules) and hydrogen peroxide (H2O2 molecules). Oxidation simulations were performed at different temperatures of 1000, 1500, 2000, 2500 and 3000 K. We found that by controlling the temperature, carbon supported titania can be formed by diffusion of Ti atoms to the surface of MXene structures during the oxidation. These results were confirmed by the increase in the average bond orders of C–C and Ti–O and decrease in the average bond orders of Ti–C. Moreover, it was revealed that by increasing the temperature, the oxidation rate increases, and the rate depends on the oxidant according to the following order: H2O2 > wet air > dry air. This was validated by heating the MXene in wet air and dry air followed by examining the products using X-ray diffraction and Raman spectroscopy. To investigate the effect of the presence of the oxidant, the MXene structure was also heated under vacuum, and it was found that in this case the MXene structure was converted to cubic TiC. Furthermore, it was indicated that during the heating process of the MXene structure in a vacuum, the average bond orders for C–C, Ti–C and Ti–O do not change significantly due to the lack of oxidants in the environment, leaving room for only topotactic transformation of atoms during the heating. The formation of the TiC structure by heating the MXene in an inert environment was confirmed experimentally as well.


 Please wait while we load your content...
Please wait while we load your content...