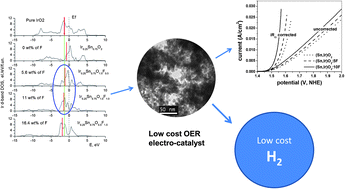
Identification and development of non-noble metal based electro-catalysts or electro-catalysts comprising compositions with significantly reduced amounts of expensive noble metal contents (e.g. IrO2, Pt) with comparable electrochemical performance to the standard noble metal/metal oxide for proton exchange membrane (PEM) based water electrolysis would signify a major breakthrough in hydrogen generation via water electrolysis. Development of such systems would lead to two primary outcomes: first, a reduction in the overall capital costs of PEM based water electrolyzers, and second, attainment of the targeted hydrogen production costs (<$3.00/gge delivered by 2015) comparable to conventional liquid fuels. In line with these goals, by exploiting a two-pronged theoretical first principles and experimental approach herein, we demonstrate for the very first time a solid solution of SnO2:10 wt% F containing only 20 at.% IrO2 [e.g. (Sn0.80Ir0.20)O2:10F] displaying remarkably similar electrochemical activity and comparable or even much improved electrochemical durability compared to pure IrO2, the accepted gold standard in oxygen evolution electro-catalysts for PEM based water electrolysis. We present the results of these studies.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...