An approach to 8 stereoisomers of homonojirimycin from d-glucosevia kinetic & thermodynamic azido-γ-lactones†
Abstract
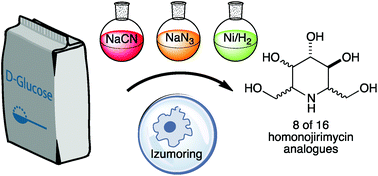
Crystal structures were obtained for the two C2 epimeric azido-γ-lactones 2-azido-2-deoxy-3,5:6,7-di-O-isopropylidene-D-glycero-D-ido-heptono-1,4-lactone and 2-azido-2-deoxy-3,5:6,7-di-O-isopropylidene-D-glycero-D-gulo-heptono-1,4-lactone prepared from kinetic and thermodynamic azide displacements of a triflate derived from D-glucoheptonolactone. Azido-γ-lactones are very useful intermediates in the synthesis of iminosugars and polyhydroxylated amino acids. In this study two epimeric azido-heptitols allow biotechnological transformations via Izumoring techniques to 8 of the 16 possible homonojirimycin analogues, 5 of which were isolated pure because of the lack of stereoselectivity of the final reductive amination. A side-by-side glycosidase inhibition profile of 11 of the possible 16 HNJ stereoisomers derived from
- This article is part of the themed collection: J400: Celebrating the 400th year of Japan-UK relations

 Please wait while we load your content...
Please wait while we load your content...