Accelerating hydrogen evolution at neutral pH by destabilization of water with a conducting oxophilic metal oxide†
Abstract
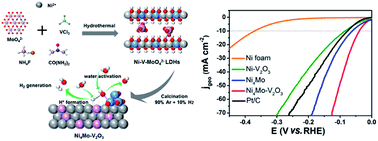
Electrolysis in neutral media is vital for cost-efficient hydrogen production utilizing vast resources like wastewater and seawater. However, the required high overpotential remains an enormous obstacle, which is due to the sluggish water dissociation Volmer step along with the subsequent proton recombination step. Herein, choosing strongly oxophilic and metallic vanadium sesquioxide (V2O3) as the water dissociation center, we present the synthesis of self-supporting Ni4Mo–V2O3 nanosheets via topotactic transformation of oxometallate intercalated layered double hydroxide (LDH). Benefiting from their good electrical conductivity, large electrochemical surface area, exposed active sites and abundant heterogeneous interfaces, Ni4Mo–V2O3 nanosheets exhibit an extremely low overpotential of 39.3 mV at 10 mA cm−2 and a Tafel slope of 65.7 mV dec−1 for the hydrogen evolution reaction (HER) at neutral pH. Density functional theory calculations confirm that V2O3 on Ni4Mo alloy enhances water adsorption and reduces the energy barriers for water dissociation, as well as the subsequent H2 generation, thus achieving superior HER performance in neutral electrolyte.


 Please wait while we load your content...
Please wait while we load your content...