Torsion effect of the imide ring on the performance of transparent polyimide films with methyl-substituted phenylenediamine†
Abstract
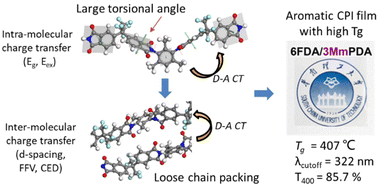
On account of the urgent demand for wearable devices and flexible displays, high transparency and heat-resistance of colorless polyimide (CPI) are required for its applicability and durability. Considering the steric hindrance and cheap source of methyl (CH3) groups, a series of CPI films were prepared from CH3-substituted diamines and 4,4′-(hexafluoroisopropylidene)diphthalic anhydride (6FDA), exhibiting greatly improved optical and thermal properties. Furthermore, the torsion effects of imide rings on the performance of CPI films were quantitatively studied by density functional theory (DFT) and molecular dynamics (MD) simulation. The steric hindrance of the ortho-CH3 moiety leads to a large torsional angle and torsional energy barrier, leading to a high glass transition temperature (Tg) and superior transparency. Via time-dependent DFT (TD-DFT), the excitation energy was calculated and it shows a highly positive correlation with the experimental transparency (T400). Particularly, an exceptional CPI film was obtained, which exhibits outstanding optical and thermal properties, including high Tg (407 °C), low cutoff wavelength (λcutoff = 322 nm), ultra-high transmittance at 400 nm (T400 = 85.7%), ultra-low yellowness (b* = 0.53) and yellow index (YI = 1.00). These systematic investigations would provide rational guidelines to develop high-performance aromatic CPI films from cheap methylated diamine monomers, as well as complementing the basic structure–property relationship of aromatic CPIs.


 Please wait while we load your content...
Please wait while we load your content...