Organic redox polymers as electrochemical energy materials
Abstract
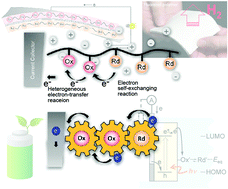
Redox polymers reversibly release electrons (undergo oxidation) and gain electrons (undergo reduction). The chemical design of organic-based redox polymers is very interesting from the perspectives of studying their unique electrochemical capabilities and their use in a variety of charge-storage-device and redox-catalyst applications. This review begins by introducing the rapid electron-exchange reactions of organic redox molecules and the bistability of their oxidised and reduced forms, followed by a discussion on efficient and reversible charge propagation and storage in redox polymers containing backbones that are densely populated with redox-active sites. Employing redox polymers in rechargeable charge-storage devices, as hydrogen carriers, and for the electrochemical production of hydrogen peroxide, hydrogen, and oxygen from water is then discussed, including more-recent examples that include flexible and high-power batteries, electrode-active biomaterials, and solar-driven catalytic films. The advantages of redox polymers are also noted in terms of non-biohazardous organic materials that are derived from relatively-abundant resources, as well as their functions in environmentally-friendly applications. This review provides basic insight into the characteristics of redox polymers, and is expected to stimulate their development as sustainable materials for use in energy-related technologies.
- This article is part of the themed collection: Green Chemistry Reviews


 Please wait while we load your content...
Please wait while we load your content...