En route to full implementation: driving the green chemistry agenda in the pharmaceutical industry†
Abstract
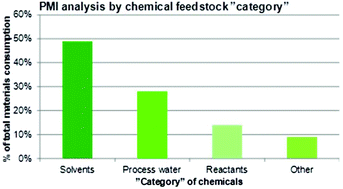
What is the relationship between the Green Chemistry initiative and the pharmaceutical industry? The intention is to shed some light on this issue by providing an historical overview spanning a period of about 20 years – from the start of the movement towards greener processes and manufacture in the early 1990s until today where greenness and sustainability are widely embraced throughout society. To understand and appreciate the approach to the green paradigm from a pharmaceutical business point of view, it is essential to paint the broader picture explaining the landscape in which this industry operates and its particular challenges. Looking at the special features that apply to chemical production of drug molecules for commercial use – in relative terms a low volume undertaking (from kg scale through to 10s or sometimes 100s of tons per annum) – the situation is vastly different compared to conventional bulk manufacture (for instance of commodity chemicals). After an initial lag phase, the drug industry has now caught up and is very eager to fully adopt green principles and to gather evidence on how it is performing. As an example, it is a well documented fact that more than half the mass constituting a process stream in the chemical manufacture of active pharmaceutical ingredients (APIs) stems from the solvent(s) utilized; 80–90% if water is included. In a multi-step synthesis on an average composed of 8–10 discrete chemical transformations which typically runs at a process mass intensity (PMI) factor of 100–200 kg kg−1 API, about 50–100 kg can be referred purely to the contribution from solvents; hence, the potential for improvements is huge. Thus, leaving the historic priorities behind in favor of drivers for change such as external pressure, goodwill, legislation, and company policies is a good strategy to ensure a rapid movement into a greener future, albeit without ignoring the existence of blockers that mainly relate to insufficient scientific and technological capabilities. From a chemical process point of view, there are several reasons to have an optimistic view about the prospects of a flourishing green agenda going forward as shown in a number of recent case studies.
- This article is part of the themed collection: Green Solvents

 Please wait while we load your content...
Please wait while we load your content...