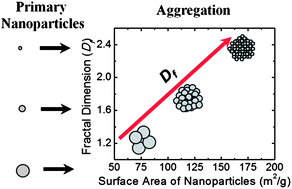
A systematic investigation was conducted to understand the role of aquatic conditions on the aggregate morphology of nano-TiO2, and the subsequent impact on their fate in the environment. In this study, three distinctly sized TiO2nanoparticles (6, 13, and 23 nm) that had been synthesized with flame spray pyrolysis were employed. Nanoparticle aggregate morphology was measured using static light scattering (SLS) over a wide range of solution chemistry, and in the presence of natural organic matter (NOM). Results showed that primary nanoparticle size can significantly affect the fractal dimension of stable aggregates. A linear relationship was observed between surface areas of primary nanoparticles and fractal dimension indicating that smaller primary nanoparticles can form more compact aggregate in the aquatic environment. The pH, ionic strength, and ion valence also influenced the aggregate morphology of TNPs. Increased pH resulted a decrease in fractal dimension, whereas higher ionic strength resulted increased fractal dimension particularly for monovalent ions. When NOM was present, aggregate fractal dimension was also affected, which was also notably dependent on solution chemistry. Fractal dimension of aggregate increase for 6 nm system in the presence of NOM, whereas a drop in fractal dimension was observed for 13 nm and 23 nm aggregates. This effect was most profound for aggregates comprised of the smallest primary particles suggesting that interactions of NOM with smaller primary nanoparticles are more significant than those with larger ones. The findings from this study will be helpful for the prediction of nanoparticle aggregate fate in the aquatic environment.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...