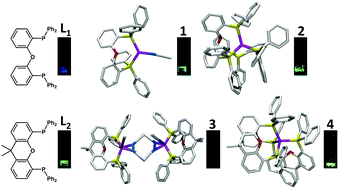
We studied the luminescence properties of copper(I) complexes containing bis[2-(diphenylphosphino)phenyl]ether (DPEphos), [Cu(DPEphos)(CH3CN)]PF6 (1), [Cu(DPEphos)2]PF6 (2), and copper(I) complexes with 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene (xantphos), [Cu(xantphos)(CH3CN)2]PF6 (3), [Cu(xantphos)2]PF6 (4) in the solid state. The metal-free xantphos ligand shows weak phosphorescent emission at around 455 nm with an emission lifetime in the sub-microsecond range in the solid state at room temperature. When xantphos forms complex 4, it results in a nearly 4-fold increase in the emission quantum yield and emission lifetime, with a small shift in the emission maximum. In contrast, no such enhancement in the luminescence or increment in the emission lifetime was observed in complexes 1–3. The X-ray structural analysis of complexes 1–4 reveals a large vacant space in complexes 1–3 and in contrast, close packing of the ligands in complex 4 around the metal center. This indicates that the decrease in the free-space around the metal center results in a decrease in the geometric relaxation, suppressing the excited-state deactivation pathway.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...