Structural changes in supercooled Al2O3–Y2O3 liquids
Abstract
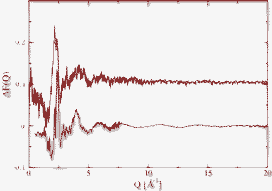
Structural changes in liquids between Al2O3 and Y2O3 are investigated as a function of the composition and during supercooling using high energy
- This article is part of the themed collection: Hard X-rays and the Frontiers of Materials Chemistry

 Please wait while we load your content...
Please wait while we load your content...