Chemometric analysis of spectroscopic data on shape evolution of silver nanoparticles induced by hydrogen peroxide†
Abstract
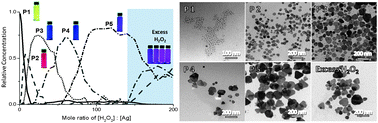
The study on the shape evolution of metal
- This article is part of the themed collection: Optical studies of single metal nanoparticles

 Please wait while we load your content...
Please wait while we load your content...