Development of a ReaxFF reactive force field for lithium ion conducting solid electrolyte Li1+xAlxTi2−x(PO4)3 (LATP)†
Abstract
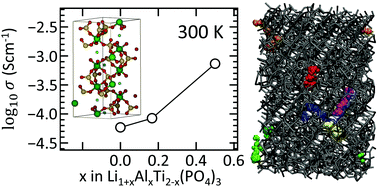
We developed a ReaxFF reactive force field for NASICON-type Li1+xAlxTi2−x(PO4)3 (LATP) materials, which is a promising solid-electrolyte that may enable all-solid-state lithium-ion batteries. The force field parameters were optimized based on density functional theory (DFT) data, including equations of state and the heats of formation of ternary metal oxides and metal phosphate crystal phases (e.g., LixTiO2, Al2TiO5, LiAlO2, AlPO4, Li3PO4 and LiTi2(PO4)3 (LTP)), and the energy barriers for Li diffusion in TiO2 and LTP via vacancies and interstitial sites. Using ReaxFF, the structural and the energetic features of LATP were described properly across various compositions – Li occupies more preferentially the interstitial site next to Al than next to Ti. Also, as observed in experimental data, the lattice parameters decrease when Ti is partly substituted by Al because of the smaller size of the Al cation. Using this force field, the diffusion mechanism and the ionic conductivity of Li in LTP and LATP were investigated at T = 300–1100 K. Low ionic conductivity (5.9 × 10−5 S cm−1 at 300 K) was obtained in LTP as previously reported. In LATP at x = 0.2, the ionic conductivity was slightly improved (8.4 × 10−5 S cm−1), but it is still below the experimental value, which is on the order of 10−4 to 10−3 S cm−1 at x = 0.3–0.5. At higher x (higher Al composition), LATP has a configurational diversity due to the Al substitution and the concomitant insertion of Li. By performing a hybrid MC/MD simulation for LATP at x = 0.5, a thermodynamically stable LATP configuration was obtained. The ionic conductivity of this LATP configuration was calculated to be 7.4 × 10−4 S cm−1 at 300 K, which is one order of magnitude higher than the ionic conductivity for LTP and LATP at x = 0.2. This value is in good agreement with our experimental value (2.5 × 10−4 S cm−1 at 300 K) and the literature values. The composition-dependent ionic conductivity of LATP was successfully demonstrated using the ReaxFF reactive force field, verifying the applicability of the LATP force field for the understanding of Li diffusion and the design of highly Li ion conductive solid electrolytes. Furthermore, our results also demonstrate the feasibility of the MC/MD method in modeling LATP configuration, and provide compelling evidence for the solid solution sensitivity on ionic conductivity.


 Please wait while we load your content...
Please wait while we load your content...
