N-doped hierarchical porous carbon derived from bismuth salts decorated ZIF8 as a highly efficient electrocatalyst for CO2 reduction†
Abstract
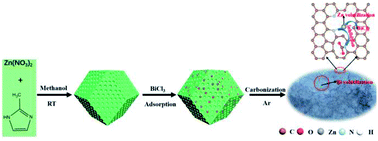
With the excessive consumption of fossil fuels, CO2 emission issues and the energy crisis have become more prominent. The electrocatalytic reduction of CO2 (ERC) driven by intermittent renewable electricity can not only mitigate carbon dioxide emission, but also store renewable energy. However, the poor activity, selectivity and stability of the catalysts inhibit their practical large-scale application. Herein, we report a facile synthesis method for a synthetic N-doped hierarchical porous carbon catalyst, which was obtained by directly carbonizing the ZIF8 precursor decorated with the low boiling metal salts (BiCl3). Plentiful bismuth salts can not only efficiently destroy the Zn–Nx bond and promote the aggregation and evaporation of the Zn species at a relatively low temperature, but also enhance the formation of a conductive and hierarchical porous catalyst. In addition, the tedious post-processing to remove the metal salts is avoided. Density functional theory calculations suggest that the coordinated unsaturated Zn–Nx sites tremendously inhibit the activity of the nearby pyridinic N sites, which play an important role in the catalytic performance. As a result, the catalysts achieve about 90% faradaic efficiency for CO at a very low overpotential of −0.49 V, which is much lower than the most reported N-doped carbon catalysts.


 Please wait while we load your content...
Please wait while we load your content...