Photocatalytic reduction of CO2 with H2O on various titanium oxide photocatalysts
Abstract
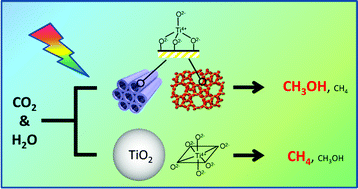
The specific features of the photocatalytic reduction of CO2 with
- This article is part of the themed collection: Carbon Dioxide
* Corresponding authors
a
Division of Materials and Manufacturing Science, Graduate School of Engineering, Osaka University, 2-1 Yamada-oka, Suita, Osaka, Japan
E-mail:
yamashita@mat.eng.osaka-u.ac.jp
Fax: +81-6-6879-7457
b
Department of Applied Chemistry, Graduate School of Engineering, Osaka Prefecture University, 1-1 Gakuen-cho, Naka-ku, Sakai, Osaka, Japan
E-mail:
anpo@chem.osakafu-u.ac.jp
Fax: +81-72-254-9941
Tel: +81-72-254-9139
The specific features of the photocatalytic reduction of CO2 with

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
K. Mori, H. Yamashita and M. Anpo, RSC Adv., 2012, 2, 3165 DOI: 10.1039/C2RA01332K
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
