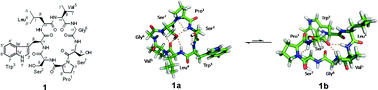
Tunicyclin E (1), a new cyclic heptapeptide, cyclo(Pro1–Ser2–Trp3–Leu4–Val5–Gly6–Ser7), was isolated from the root of Psammosilene tunicoides. The presence of two sets of resonance signals in its NMR spectra (1a:1b, ∼3 : 1 abundance) indicated that it has two conformations in solution, while only one conformation was found in its crystal state by X-ray diffraction. To explore the molecular basis of the two conformations of 1 in solution and their interconversion mechanism, X-ray diffraction, NMR experiments, and theoretical calculations were performed. The results disclosed that two conformers of 1 in solution were derived from the cis/trans isomers of the Ser7–Pro1peptide bond (1a, trans; 1b, cis). The fast interconversion of the two conformations in solution is explained by an intramolecular catalysis mechanism and solvent effects. Furthermore, the existence of several unusual pseudo turns characterized for the first time plays a key role for dominant trans conformation in solution.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...