Enhanced photocatalytic activity of g-C3N4 for selective CO2 reduction to CH3OH via facile coupling of ZnO: a direct Z-scheme mechanism
Abstract
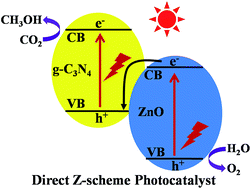
Photocatalytic CO2 reduction into renewable hydrocarbon solar fuels is considered as a promising strategy to simultaneously address the global energy and environmental issues. In this study, a binary g-C3N4/ZnO photocatalytic system was constructed via a one-step facile calcination method and further used as a photocatalyst for CO2 reduction. It was shown that the as-prepared g-C3N4/ZnO photocatalytic system exhibited enhanced photocatalytic activity for CO2 reduction by a factor of 2.3 compared with pure g-C3N4, while maintaining the original selectivity of pure g-C3N4 to convert CO2 directly into CH3OH. For the first time, the coupling effect of ZnO responsible for the improved photoactivity of g-C3N4 was fully illustrated and a direct Z-scheme mechanism rather than the conventional heterojunction-type mechanism was proposed to explain the better performances of the g-C3N4/ZnO binary composite photocatalytic system. The enhancement of photocatalytic CO2 reduction activity is attributed to the highly efficient ZnO-to-g-C3N4 electron transfer occurring at the intimate contact interface between the g-C3N4 phase and ZnO phase. This work will provide new deep insights into the rational construction of a g-C3N4-based photocatalytic system and the design of a direct Z-scheme system without an electron mediator for photocatalytic CO2 reduction reactions.

 Please wait while we load your content...
Please wait while we load your content...