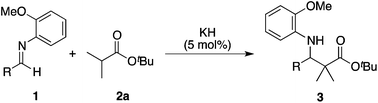
Development of strong Brønsted base catalysis: catalytic direct-type Mannich reactions of non-activated estersvia a product-base mechanism†‡
Abstract
A catalytic
- This article is part of the themed collection: Organic & Biomolecular Chemistry 10th Anniversary

 Please wait while we load your content...
Please wait while we load your content...