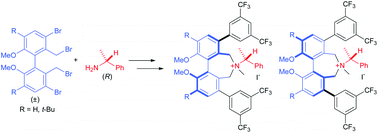
Synthesis and evaluation of atropos dihydro-5H-dibenzazepinium halide PTCs derived from α-methylbenzylamine†‡
Abstract
A short synthetic route to diastereoisomeric atropos dihydro-5H-dibenz[c,e]azepinium salts via reaction of a single enantiomer of
- This article is part of the themed collection: Organocatalysis
 Please wait while we load your content...
Please wait while we load your content...