Structure, stereochemistry and synthesis of enantiopure cyclohexenone cis-diol bacterial metabolites derived from phenols†‡
Abstract
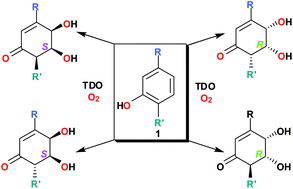
Biotransformation of 3-substituted and 2,5-disubstituted phenols, using whole cells of P. putida UV4, yielded cyclohexenone cis-diols as single enantiomers; their structures and absolute configurations have been determined by NMR and ECD spectroscopy, X-ray crystallography, and stereochemical correlation involving a four step chemoenzymatic synthesis from the corresponding cis-dihydrodiol metabolites. An active site model has been proposed, to account for the formation of enantiopure cyclohexenone cis-diols with opposite absolute configurations.
- This article is part of the themed collection: Organic & Biomolecular Chemistry 10th Anniversary

 Please wait while we load your content...
Please wait while we load your content...