Direct amination of α-substituted nitroacetates using di-tert-butyl azodicarboxylate catalyzed by Hatakeyama's catalyst β-ICD†‡
Abstract
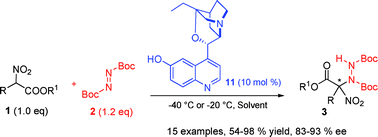
We report the first example of catalytic asymmetric direct
- This article is part of the themed collection: Organocatalysis

 Please wait while we load your content...
Please wait while we load your content...