Innate pharmacophore assisted selective C–H functionalization to therapeutically important nicotinamides†
Abstract
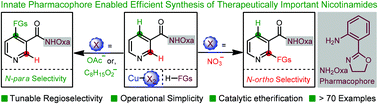
The application of the pre-validated pharmacophore 2-(2-oxazolinyl)aniline as an innate directing group in the C–H etherification and amination of nicotinamides for the efficient synthesis of drug- and agrochemical-like molecules was determined. An operationally simple, and regioselective C–H functionalization of nicotinamides was first accomplished using a complicated variation of copper salts. All the specific procedures used were easy to utilize, without external oxidants or ligands. The feasibility is highlighted using an alternative synthesis of diflufenican with a rapid synthesis of niacin related pharmaceuticals or analogues.


 Please wait while we load your content...
Please wait while we load your content...