Optimized Li and Fe recovery from spent lithium-ion batteries via a solution-precipitation method
Abstract
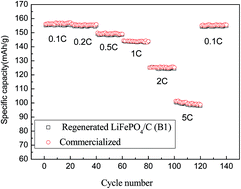
A new process is optimized and presented for the recovery and regeneration of LiFePO4 from spent lithium-ion batteries (LIBs). The recycling process reduces the cost and secondary pollution caused by complicated separation and purification processes in spent LIB recycling. Amorphous FePO4·2H2O was recovered by a dissolution-precipitation method from spent LiFePO4 batteries. The effects of different surfactants (i.e. CTAB, SDS and PEG), which were added to the solution on the recovered FePO4·2H2O, were investigated. Li2CO3 was precipitated by adding Na2CO3 to the filtrate. Then the LiFePO4/C material was synthesized by a carbon thermal reduction method using recycled FePO4·2H2O and Li2CO3 as the Fe, P, and Li sources. The as-prepared LiFePO4/C shows comparable electrochemical performance to that of commercial equivalents.

 Please wait while we load your content...
Please wait while we load your content...