Nanostructured nickel manganese oxide: aligned nanostructures and their magnetic properties
Abstract
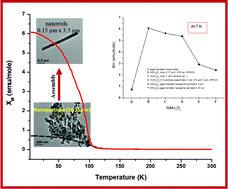
The present study describes the control of the assembly of NiMn2O4 nanospheres and hexagonal particles by tuning the precursor (nickel manganese oxalate) morphology. The aspect ratio (2 to 24) of the nickel manganese oxalate precursors has been achieved by the variation of the
- This article is part of the themed collection: Australia-India Joint Symposium on Smart Nanomaterials

 Please wait while we load your content...
Please wait while we load your content...