Heterolytic activation of hydrogen using frustrated Lewis pairs containing tris(2,2′,2′′-perfluorobiphenyl)borane†
Abstract
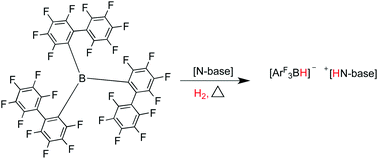
The extremely sterically hindered borane tris(2,2′,2′′-perfluorobiphenyl)borane (PBB) has been structurally characterised. In combination with bulky nitrogen bases, it forms the ‘frustrated Lewis pairs’ (FLPs) PBB/2,2,6,6-tetramethylpiperidine (TMP) (1), PBB/1,4-diazobicyclo[2.2.2]-octane (DABCO) (2) and PBB/2,6-lutidine (lut) (3). These novel, unquenched acid–base pairs have been shown to effect facile room temperature heterolytic cleavage of dihydrogen to form the ammonium borate salts [2,2,6,6-Me4C5H6NH2][HB(C12F9)3] (4) and [N(C2H4)3NH][HB(C12F9)3] (5), and lutidinium borate [2,6-Me2C5H3NH][HB(C12F9)3] (6). Although these reactions are equilibria, the reverse reaction and release of hydrogen gas was not apparent at temperatures up to 120 °C. The relative Lewis acidity of PBB has been determined using the Gutmann–Beckett method.
- This article is part of the themed collection: Frustrated Lewis Pairs

 Please wait while we load your content...
Please wait while we load your content...