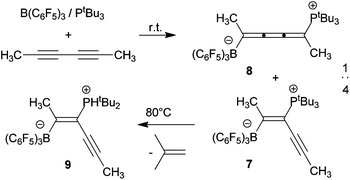
The frustrated Lewis pair B(C6F5)3/P(o-tolyl)3 (4a) reacts with 4,6-decadiyne to give the trans-1,2-addition product 5. In contrast, the B(C6F5)3/PtBu3 FLP (4b) reacts with this substrate to give the trans-1,4-adduct trans-6. The cumulene trans-6 undergoes trans-/cis-isomerization upon photolysis to give a ca. 1 : 1 trans-6/cis-6 mixture. The FLP 4b reacts with 2,6-hexadiyne at r.t. to yield a ca. 4 : 1 mixture of their trans-1,2- and trans-1,4-addition products (7,8). DFT calculations showed that the zwitterionic 1,4-addition products are favored under thermodynamic control. Thermolysis of the kinetic trans-1,2-addition product (7) (80 °C, bromobenzene) does not lead to the thermodynamically favored 1,4-isomer (8), but instead elimination of isobutylene occurs to the formal trans-1,2-adduct (9) of the B(C6F5)3/PHtBu2 pair. Compounds 5, 6, 7, 8, 9 were analyzed by X-ray diffraction.