Abstract
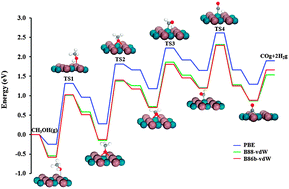
Methanol decomposition to CO and H2 on PdIn(110) has been studied by following the sequential dehydrogenation steps from CH3OH → CH3O → CH2O → CHO → CO using density functional theory slab calculations. The first three of the four elementary steps are strongly endothermic. The last step, i.e., CHO → CO + H, is almost thermal neutral. We also examined the effect of considering van der Waals interaction on the reaction energy and activation barrier of each elementary step by using the optB88-vdW and optB86b-vdW functionals. Our results show that both overall reaction energy and activation barrier were reduced by including van der Waals interactions but the qualitative picture remains unchanged.
- This article is part of the themed collection: Computational Catalysis and Materials for Energy Production, Storage and Utilization

 Please wait while we load your content...
Please wait while we load your content...