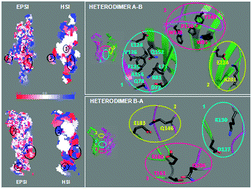
The functional serotonin type-3 receptor (5-HT3-R), which is the target of many neuroactive drugs, is known to be a homopentamer made of five identical subunits A (5-HT3A-R) or a binary heteropentamer made of subunits A and B (5-HT3A/B-R) with a still debated arrangement and stoichiometry. This complex picture has been recently further complicated by the discovery of additional 5-HT3-R subunits, C, D, and E, which, similarly to the B subunit, are apparently able to form functional receptors only if co-expressed with subunit A. Being the binding site for both serotonin and antagonists (i.e. drugs) located at the extracellular interface between two adjacent subunits, the large variability of the 5-HT3-R composition becomes a crucial issue, since it can originate many different interfaces providing non-equivalent ligand binding sites and complicating the pharmacological modulation. Here, the different 5-HT3-R interfaces are analysed, on the bases of the structural conformations of previously built 3D homology models and of the known subunit sequences, by addressing their physicochemical characterization. The results confirm the presence of an aromatic cluster located in the core of the A–A interface as a key determinant for having an interface both stable and functional. This is used as a discriminant to make hypotheses about the capability of all the other possible interfaces constituted by the known 5-HT3-R sequences A, B, C, D, and E to build active receptors.

 Please wait while we load your content...
Please wait while we load your content...