Effect of hydrogen-bonding on the excited-state reactivity of fullerene derivatives and its impact on the control of the emission polarisation from photopolic single crystals
Abstract
The kinetics of the irreversible photoinduced switch in polarisation (p) observed in single crystals of a
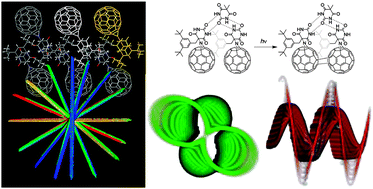
- This article is part of the themed collection: Hydrogen bonding in electronically excited states

 Please wait while we load your content...
Please wait while we load your content...