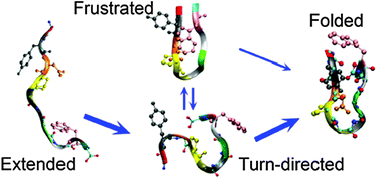
Realistic mechanistic pictures of β-hairpin formation, offering valuable insights into some of the key early events in protein folding, are accessible through short designed polypeptides as they allow atomic-level scrutiny through simulations. Here, we present a detailed picture of the dynamics and mechanism of β-hairpin formation of Chignolin, a de novo decapeptide, using extensive, unbiased molecular dynamics simulations. The results provide clear evidence for turn-directed broken-zipper folding and reveal details of turn nucleation and cooperative progression of turn growth, hydrogen-bond formations, and eventual packing of the hydrophobic core. Further, we show that, rather than driving folding through hydrophobic collapse, cross-strand side-chain packing could in fact be rate-limiting as packing frustrations can delay formation of the native hydrophobic core prior to or during folding and even cause relatively long-living misfolded or partially folded states that may nucleate aggregative events in more complex situations. The results support the increasing evidence for turn-centric folding mechanisms for β-hairpin formation suggested recently for GB1 and Peptide 1 based on experiments and simulations but also point to the need for similar examinations of polypeptides with larger numbers of cross-strand hydrophobic residues.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...