The effect of hydrogen bonding on the excited-state proton transfer in 2-(2′-hydroxyphenyl)benzothiazole: a TDDFT molecular dynamics study†
Abstract
The dynamics of the excited-state proton transfer (ESPT) in a cluster of
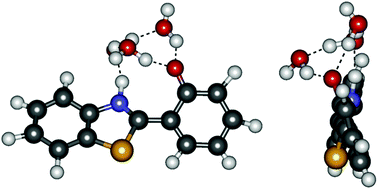
- This article is part of the themed collection: Hydrogen bonding in electronically excited states

 Please wait while we load your content...
Please wait while we load your content...