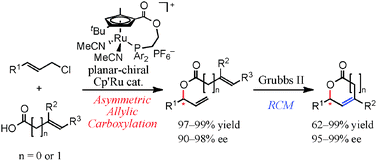
Modular synthesis of optically active lactones by Ru-catalyzed asymmetric allylic carboxylation and ring-closing metathesis reaction†
Abstract
A new synthetic route to optically active unsaturated γ- and δ-lactones has been demonstrated via asymmetric allylic
- This article is part of the themed collection: Chirality

 Please wait while we load your content...
Please wait while we load your content...