Mechanistic studies on millerite chlorination with ammonium chloride†
Abstract
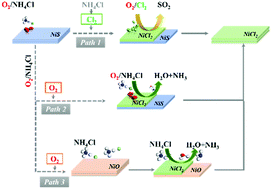
Millerite (NiS) is the main source for metallurgical production of nickel worldwide. To improve the extraction rate of nickel, chlorination is usually carried out, as the resulting nickel chloride (NiCl2) can easily dissolve in water and be separated. Although molecular chlorine (Cl2) can be used as the chlorination reagent, greener reagents such as ammonium chloride (NH4Cl) are preferable from a process design perspective. However, the efficiency of NH4Cl as a chlorination reagent must be further improved for this process to be viable for industrial applications, and mechanistic understanding is imperative to this end. Here, we performed extensive density functional theory (DFT) calculations to elucidate the chlorination mechanism of NiS by exploring three possible pathways. We first considered the direct chlorination of NiS by Cl2, which was suggested to form by the reaction between NH4Cl and SO3 catalyzed by a metal oxide. Alternatively, NH4Cl was found to react favorably with the partially or fully oxidized NiS surface in the presence of oxygen (O2). During the oxidation of NiS, sulfur dioxide (SO2) may form. Furthermore, sulfur or oxygen vacancy was predicted to form during the chlorination of NiS or NiO with NH4Cl. Based on the available experimental evidence and our computational results, three possible mechanisms for the chlorination of NiS using NH4Cl as the chlorination reagent in the presence of O2 were proposed.


 Please wait while we load your content...
Please wait while we load your content...