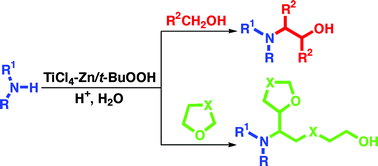
We report a new and fast domino synthesis of aminoalcohols under mild conditions. The free-radical reaction of aliphatic and aromatic amines with alcohol cosolvents is promoted by means of the TiCl4–Zn/t-BuOOH system. According to the proposed mechanism, the amine reacts with two molecules of alcohol in an electrophilic–nucleophilic cascade process. This procedure, if compared with the TiCl3/t-BuOOH-mediated protocol previously reported, appears to be more selective, of more general applicability and affords the desired products in higher yields. Besides, with the same catalytic system it was possible to promote the reaction of primary arylamines with two molecules of cyclic ether, leading to the formation of a wider range of functionalized aminoalcohols.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...